In Stem Cell Research

Revolutionizing Liquid Biopsy
High-Yield Isolation of sEVs for Research, Early Disease Diagnostics and Treatment Strategies.
Acoustofluidics, fusion of acoustics and microfluidics, is a world leading technology platform developed at Penn State University and Duke University.
It has many advantages that competing techniques lack: label and marker-free, high integrity, versatility for applications and sample volume, compact devices, fast and effective fluid actuation, contact-free and non-invasive particle/cell manipulation, and integration with other lab-on-a-chip functions.
We introduce “acoustic tweezers,” an active patterning technique using standing surface acoustic waves (SSAW) to manipulate cells and microparticles regardless of their characteristics. With power intensity significantly lower than optical tweezers and proven non-invasiveness, this simple, miniaturizable technique holds promise for applications in biology, chemistry, engineering, and materials science.
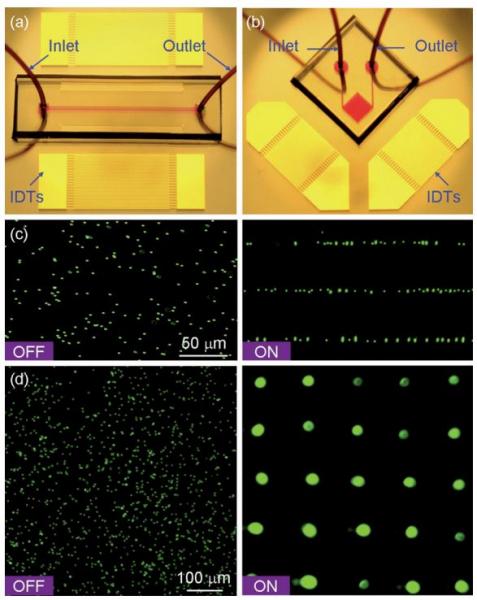
Figure: Patterning of fluorescent polystyrene microbeads. Optical images of the ‘‘acoustic tweezers’’ devices used in (a) 1D and (b) 2D patterning experiments, respectively. (c) Distribution of the microbeads before and after the 1D patterning process. The microchannel (width 150 μm and depth 80 μm) covered three lines of pressure nodes of the generated SSAW. The wavelength of SAW was 100 mm (d), Distribution of the microbeads before and after the 2D patterning process. The SAW wavelength was 200 μm.
We demonstrate “acoustic tweezers” based on standing surface acoustic waves for manipulating single microparticles, cells, and organisms like Caenorhabditis elegans in a microfluidic chip. With real-time control, low power density, and proven biocompatibility, these acoustic tweezers offer versatile, miniaturizable, and gentle manipulation, making them a promising tool across various scientific and engineering disciplines.
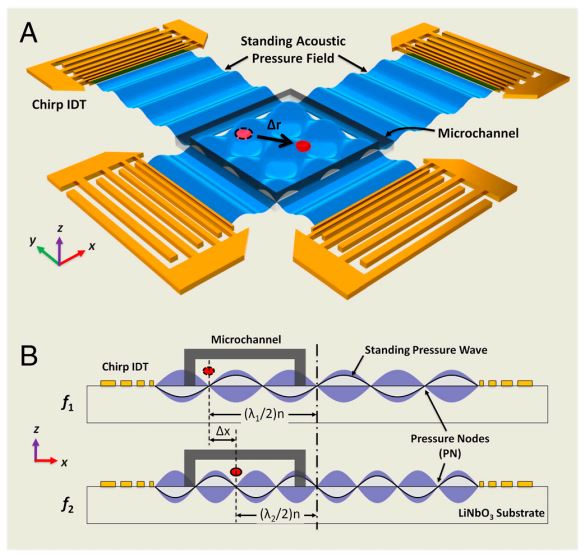
Figure: Device structure and working mechanism of the acoustic tweezers. (A) Schematic illustrating a microfluidic device with orthogonal pairs of chirped IDTs for generating standing SAW. (B) A standing SAW field generated by driving chirped IDTs at frequency f 1 and f2. When particles are trapped at the nth pressure node, they can be translated a distance of (Δλ∕2) n by switching from f1 to f2. This relationship indicates that the particle displacement can be tuned by varying the pressure node where the particle is trapped.
We demonstrate rapid and homogeneous mixing in a microfluidic channel using acoustic streaming induced by oscillating sidewall sharp-edges. Optimizing the sharp-edge design enables excellent mixing performance and speed in a simple device, making our acoustic micromixer a promising candidate for various applications.

Figure: (a) Schematic of the sharp-edge-based acoustofluidic mixing device. This device includes a PDMS microfluidic channel and a piezoelectric transducer. (b) Schematic showing the acoustic streaming phenomenon around the tip of an acoustically oscillated sharp-edge. (c) Schematic showing the design of the channel and sharp-edge.
We present a programmable acoustofluidic pump that uses acoustic streaming from oscillating tilted sharp-edge structures to generate stable flow rates up to 8 μL/min (~76 Pa pumping pressure). With a wide tuning range and minimal hardware requirements, this easy-to-operate pump shows great potential for various applications.
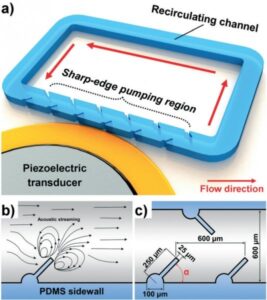
Figure: (a) Schematic of the sharp-edge-based acoustofluidic pumping device. This device includes a PDMS microfluidic channel and a piezoelectric transducer. (b) Schematic showing the acoustic streaming phenomenon around the tip of a tilted oscillating sharp-edge structure. (c) Schematic showing the design of the channel and sharp-edge structure.
We present an acoustic-based method for precise on-chip rotation of cells and organisms. Using trapped microbubbles to create microvortices in an acoustic field, we can rotate samples like C. elegans. This compact, biocompatible technique allows rotation regardless of the sample’s properties.
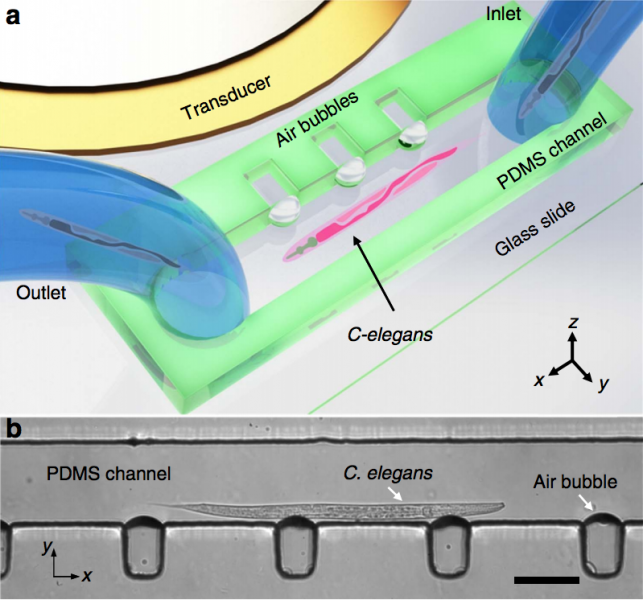
Figure: Design and operation of the acoustofluidic rotational manipulation (ARM) device. (a) A schematic of the experimental setup. The piezoelectric transducer that generates acoustic waves is placed adjacent to the microfluidic channel. The acoustic waves actuate air microbubbles trapped within sidewall microcavities. (b) An optical image showing a mid-L4 stage C. elegans trapped by multiple oscillating microbubbles. Scale bar, 100 microns.
We present an acoustofluidic technique for creating programmable chemical waveforms, enabling precise control over signal characteristics and rapid switching between stimuli. This approach facilitates detailed studies of biological responses, such as receptor activation, and has potential to advance research in dynamic biological processes.
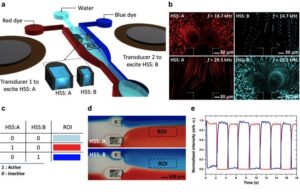
Figure: Bubble-based switching between multiple stimuli. (a) Schematic of the experimental setup for chemical switching. The microfluidic channel contains HSSs of different sizes and, subsequently, bubbles of different sizes that are independently driven by transducers bonded to the substrate adjacent to the channel. (b) Top, visualization of microstreaming from the bubble trapped in HSS A (red) while no streaming is observed in the bubble trapped in HSS B (blue) at an excitation frequency of 14.7 kHz. Bottom, visualization of the microstreaming from the bubble trapped in HSS B while no streaming occurs in HSS A at an excitation frequency of 29.5 kHz. (c) Table showing the concept of binary logic circuitry. (d) Results demonstrating switching between the blue and red dyes. (e) Graph of experimental data for switching between red and blue dyes in the selected ROI marked in panel d.
We report on a portable system for controlling acoustofluidic devices using a cell phone and a modified Bluetooth® speaker, enabling on-demand, hands-free pumping and mixing. Additionally, we propose a novel design for a device that combines both functions, eliminating the need for external equipment. These advancements demonstrate the potential of acoustofluidic devices in point-of-care platforms.
Figure (a) Comparison between conventional acoustofluidic device operation and portable operation. The portable control platform can be used with either a traditional benchtop microscope or a portable microscope based on a cell phone camera. Not to scale. (b) Photo of the cell phone, modified speaker and acoustofluidic device. Signal measured from the speaker (c) before and (d) after passing through the RLC filter circuit to increase the voltage.

We present a plasmofluidic lens that combines plasmonics and microfluidics, enabling dynamic tuning and reconfiguration of plasmonic lenses. This innovation can manipulate light at the nanoscale and paves the way for advanced plasmonic elements with diverse functionalities, suitable for biomedical detection and optical information processing.
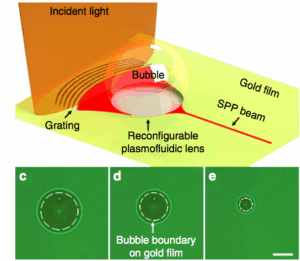
Figure: Schematic of the reconfigurable plasmofluidic lens, wherein a laser-induced surface bubble is used to control the propagation of SPPs at the metal surface. (c–e) Surface bubbles with different diameters (18, 14 and 6 microns, respectively) generated on the gold film. The white dashed circle represents the surface bubble boundary on gold film. Scale bar, 10 microns.
We describe a tunable optofluidic microlens formed by fluids with different refractive indices in a curved microchannel. The lens’s optical properties are adjustable by changing the flow rate, affecting its curvature and focal length. This mechanism allows for the integration of adaptable microlenses into lab-on-a-chip systems, confirmed through various simulations and microscopy.
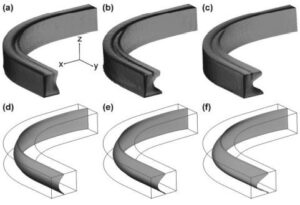
Figure: The 3D architectures of water flow obtained with confocal microscopy at the flow rates of (a) 100 ml min21 , (b) 150 ml min21 , and (c) 250 ml min21 . Images in (d–f) show the corresponding CFDsimulated 3D fluidic interfaces (isosurface of CaCl2 concentration = 2.5 M).
Enabling Breakthrough Discoveries
Bridging the Gap Between Research and Real-World Treatments
Acoustofluidics opens new avenues of research through the rare combination of sound waves and fluids, mechanics and electronics, experiment and analysis, physics and materials, and engineering and biomedicine.
In Stem Cell Research

In Pregnancy Complexity

In Drug Development

In Disease Diagnostics

In Cancer Research
In Asthma Research
In Alzheimer’s Research

